
Reserch
Institute for Molecular and Cellular Regulation
Molecular Endocrinology and Metabolism

Ken Sato Professor
To understand the molecular mechanisms and physiological functions of membrane trafficking in multicellular organisms, we use the nematode Caenorhabditis elegans and mice as model systems.
Research and Education
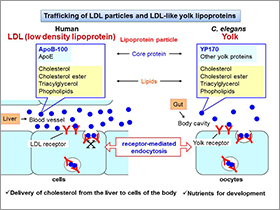
1) Molecular mechanisms of LDL trafficking in C. elegans
LDL is a low density lipoprotein consisting of core proteins and lipids such as cholesterol. LDL is recognized by the LDL receptor on the cell surface and then taken up by cells via receptor-mediated endocytosis. This process is also important to remove LDL from blood and keep a normal level of LDL in the blood. Interestingly, C. elegans yolk has a very similar character to mammalian LDL. In C. elegans, yolk is taken up by oocytes via receptor-mediated endocytosis (Figure, upper panel). In order to study LDL endocytosis in multicellular organisms, we utilize advanced genetics available in C. elegans. We are studying endocytosis-defective mutants of C. elegans to identify novel components required for LDL endocytosis.
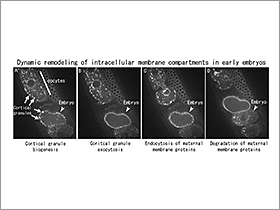
2郢晢スサ鬮ッ・ーnalysis of physiological functions and molecular mechanisms of membrane trafficking during development.
C. elegans is emerging as an amenable model system for the study of oogenesis, fertilization and embryogenesis because all of these processes can be observed easily in a living animal. We have identified a novel type of developmentally-regulated secretory granules in C. elegans oocytes (Figure, lower panel). These vesicles undergo synchronous fusion with the plasma membrane just after fertilization as has been reported for cortical granules in other animals. We are trying to clarify the molecular mechanisms of the biogenesis and exocytosis of the cortical granules as a model of the regulated secretion. Recently, we have revealed that fertilization-induced autophagy is responsible for selective degradation of paternal mitochondria and thereby maternal inheritance of mitochondrial DNA. We are now studying the molecular mechanism of paternal mitochondria degradation by fertilization-induced autophagy.
3) Analysis of physiological functions of membrane trafficking in mammal.
To study physiological functions of membrane trafficking in mammal, we are making knockout mice of the genes, which we have identified by C. elegans genetics. We are going to investigate the mutants by various cell biological methods.
 |
 |
Recent Publications
- Sato M, Sato K. (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 334, 1141-1144.
- Sato K, Ernstrom GG, Watanabe S, Weimer RM, Chen CH, Sato M, Siddiqui A, Jorgensen EM, Grant BD. (2009) Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc. of Nat. Acad. Science USA. 106, 1139-1144.
- Sato M, Grant BD, Harada A, Sato K. (2008) Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J Cell Sci 121, 3177-3186.
- Sato M, Sato K*, Liou W, Pant S, Harada A, Grant BD. (2008) Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J 27, 1183-1196.
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji Y, Harada R, and Harada A. (2007) The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366-369.
- Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. (2006) Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol. Biol. Cell. 17, 3085-3094.
- Sato M, Sato K, Fonarev P, Liou W, and Grant BD. (2005) Caenorhabditis elegans RME-6, a novel regulator of Rab5 at the clathrin-coated pit. Nature Cell Biol. 7, 559-569.

