
Reserch
Institute for Molecular and Cellular Regulation
Molecular Endocrinology and Metabolism

Tetsuro Izumi Professor
We aim to study basic biological phenomena that are deeply related with the pathogenesis of diabetes, obesity, and associated diseases. To study these subjects from a molecular level to a whole body level, we are using multidisciplinary techniques of morphology, biochemistry, molecular biology, and genetics. One of our major research areas is regulated exocytosis, which is instrumental for cell-cell communication in multicellular organisms. We are particularly focusing on insulin granule exocytosis in pancreatic beta cells, the impairment of which instantly leads to hyperglycemia. Another subject is fat accumulation in adipocytes, which is of course central to the development of obesity. Furthermore, we have recently begun to study immune cells, the disorder of which is involved in insulin resistance and chronic inflammation associated with metabolic diseases.
Research and Education
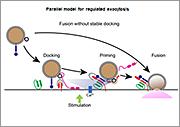
1) Relationship between docking and fusion of secretory granules
We demonstrated that the Rab27 effector, granuphilin, is essential for the stable attachment of secretory granules to the plasma membrane. Granuphilin-null secretory cells lack stably docked granules but surprisingly exhibit enhanced exocytosis in response to secretory stimulation. This finding challenges the widely believed sequential model or the linear docking-priming-fusion model that stable docking is a necessary first step before a vesicle acquires fusion competence (priming). It may also contradict the general view that a readily releasable pool derives from a subset of stably docked secretory vesicles. By directly visualizing docking, priming, and fusion of secretory granules under total internal reflection fluorescence (TIRF) microscopy in living cells expressing multiple fluorescence-labeled proteins, we have recently found that stable docking is neither a prerequisite nor a dead-end for fusion, but instead represents a temporal fusion-reluctant state to inhibit spontaneous fusion, whereas priming is not a step to acquire fusion competence before stimulation but instead occurs after sensing a stimulus-induced Ca2+ increase.
2) Mechanism for regulated exocytosis of secretory granules
The small GTPase Rab family regulates the intracellular trafficking of vesicle membrane. Among more than 60 members of mammalian Rab proteins, we showed that the small GTPases Rab27a and Rab27b and their effector proteins specifically function in regulated secretory pathways. There are 11 known Rab27 effectors (exophilin1-9, Noc2, Munc13-4), each of which is thought to play a unique role in differentiated secretory cells. We are investigating the function of Rab27 effectors at the molecular and cellular levels as well as at the whole body level using gene-knockout mice.
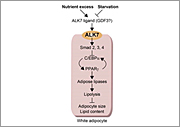
3) Genetic analysis of diabetes and obesity in rodent models
Through positional cloning of the gene loci affecting glucose tolerance and/or body weight in rodent disease models, we identified that mutation of a cysteine residue involving a disulfide bond formation in insulin molecule results in severe diabetes, whereas mutation of ALK7, one of the type I TGF beta receptors, leads to reductions in body weight and adipose mass under nutrient excess condition. Using these mouse models, we investigate the molecular mechanism of pancreatic beta-cell dysfunction and abnormal fat accumulation.
 |
 |
 |
| Parallel model for regulated exocytosis | Rab27 effector family proteins | ALK7 accumulates fat in adipocytes under nutrient excess |
Molecular Endocrinology and Metabolism

Professor: Tetsuro IZUMI
Associate Professor: Katsuhide OKUNISHI
Assistant Professor: Kohichi MATSUNAGA
Assistant Professor: Kouichi MIZUNO
Technical Officer: Takeshi USHIGOME
Phone: +81-27-220-8856
FAX: +81-27-220-8860
E-mail: tizumi@gunma-u.ac.jp
Home page: http://molend.showa.gunma-u.ac.jp/ ![]()
Recent Publications
- Yogosawa S, Mizutani S, Ogawa Y, and Izumi T (2013). Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor γ and C/EBPα. Diabetes, 62, 115-123.
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, and Shah NM (2012). Modular genetic control of sexually dimorphic behaviors. Cell, 148, 596-607.
- Kasai K, Fujita T, Gomi H, and Izumi T (2008). Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic, 9, 1191-1203.
- Gomi H, Mizutani S, Kasai K, Itohara S, and Izumi T (2005). Granuphilin molecularly docks insulin granules to the fusion machinery. J. Cell. Biol., 171, 99-109.
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, and Izumi T (2005). Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Invest., 115, 388-396.
- Wang J, Takeuchi T, Tanaka S, Kubo S-K, Kayo T, Lu D, Takata K, Koizumi A, and Izumi T (1999). A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J. Clin. Invest., 103, 27-37.
