
Reserch
Institute for Molecular and Cellular Regulation
Laboratory of Molecular Genetics

I. YAMASHITA GROUP
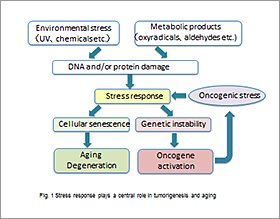
Cells are constantly exposed to a wide variety of stresses, from the environment (e.g. UV) and cell metabolism (e.g. reactive oxygen species), causing DNA and protein damages. While diverse mechanisms protect against these molecular damages, strong and/or prolonged stresses promote irreversible growth arrest (cellular senescence), cell death and genomic instability. These stress responses play critical roles for aging-related disorders and tumor development in a tightly linked manner. For instance, activated oncogenes generate “oncogenic stress” signals, which cause cellular senescence/tumor suppression as well as genetic instability/tumor progression (Fig. 1). Furthermore, since tumor cells take advantage of stress responses for their survival, control of the stress responses would open a new avenue to developing cancer therapeutics. Our current research focuses on oncogenic stress-induced genomic instability and HSF1-mediated regulation of cellular senescence.
CURRENT PROJECTS
(1) Oncogenic stress-induced genomic instability
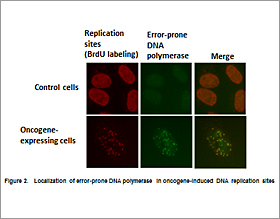
Oncogene-induced abnormal DNA replication and subsequent DNA damage promote genomic instability through poorly understood mechanisms. We previously reported that the “cancer chaperone” Hsp90 activates error-prone Y-family DNA polymerases, potentially promoting genomic instability in tumor cells. We recently found that these polymerases participate in the oncogene-induced aberrant replication (Fig. 2).
(2) Heat Shock Factor (HSF) 1-mediated regulation of cellular senescence
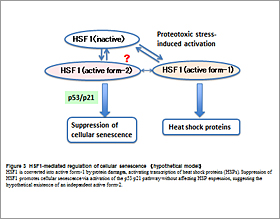
HSF1 transcriptionally activates “Heat Shock Response”, after exposure to protein-damaging stress. We recently found that acute depletion of HSF1 induces cellular senescence in non-stressed cells through activation of the p53/p21 pathway. Interestingly, HSF1 depletion has little effects on expression of heat shock proteins. These findings suggest that HSF1 regulates p53/p21-dependent cellular senescence, independently of the heat shock response (Fig. 3).
 |
 |
 |
 |
RECENT MAIN PUBLICATIONS
- Yamashita T et al. Translesion DNA synthesis and Hsp90. Genes and Environment. 2012; 34: 89-93
- Mayca Pozo F et al, Molecular chaperone Hsp90 regulates REV1-mediated mutagenesis. Mol Cell Biol 2011; 31: 3396-3409
- Sekimoto T et al, The molecular chaperone Hsp90 regulates accumulation of DNA polymerase η at replication stalling sites in UV-irradiated cells. Mol. Cell 2010; 37:79-89
- Oda T et.al, Hsp90 regulates the Fanconi anemia DNA damage response pathway. Blood 2007; 109: 5016-5026
